Treating sickle cell pain deep in the brain
A new, noninvasive, focused-ultrasound method of neuromodulation could provide an alternative to opioid pain treatment for patients with sickle cell disease by targeting the brain.
A team of Carnegie Mellon researchers, led by Bin He, department head of biomedical engineering (BME), has been awarded a National Institutes of Health Helping to End Addiction Long-Term (NIH HEAL) grant to develop a treatment for sickle cell disease that uses focused ultrasound neuromodulation, a noninvasive, non-toxic, non-addictive alternative to the use of opioid pain medications.
Sickle cell disease (SCD) is a group of genetic disorders inherited at birth that cause red blood cells to break down and become misshapen. In SCD, red blood cells, or the cells that carry oxygen throughout the body, contain an abnormal protein that causes the cells to distort into a sickle or crescent shape. When red blood cells become sickled, the cells die early, leaving a shortage of healthy red blood cells. This shortage can cause a blockage of blood flow and a significant amount of pain, fatigue, and other serious problems, such as infection and strokes.
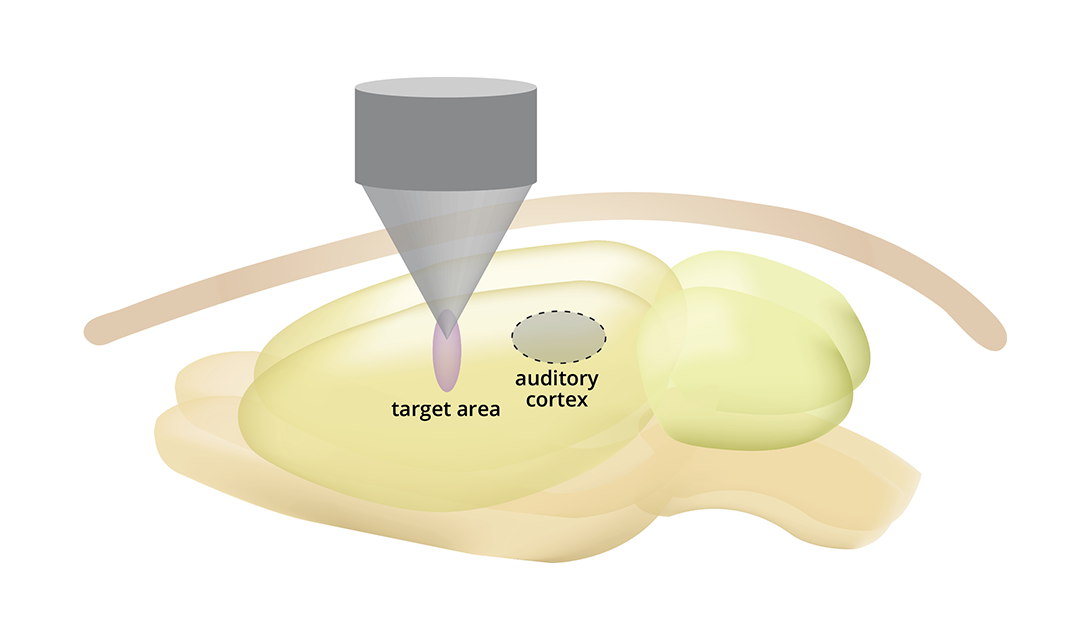
Source: College of Engineering
Schematic illustration of transcranial focused ultrasound neuromodulation treating pain by targeting brain networks responsible for pain.
SCD is a widespread affliction, affecting millions of people worldwide with chronic and acute pain. This pain can start during infancy and increase in severity throughout life, and patients often require recurrent hospitalization and long-term use of opioid medications, decreasing patients’ quality of life. Opioids are highly addictive pain relievers that can have adverse side effects and lead to addiction. Every day, more than 130 people in the United States die after overdosing on opioids.
The project proposes to develop and validate a novel focused ultrasound device that will allow for the noninvasive targeting and stimulating of brain regions with high focality and depth penetration, in order to treat SCD pain and provide a much-needed alternative to opioids.
“There is an urgent unmet need to develop safe, effective, and non-addictive device-based technologies to treat pain in sickle cell disease,” said He. “We believe a noninvasive neuromodulation method is the answer. Through innovation in engineering methodology, such a noninvasive pain management device would revolutionize current clinical practices in pain management.”
There is an urgent unmet need to develop safe, effective, and non-addictive device-based technologies to treat pain in sickle cell disease.
Bin He, Department Head, Biomedical Engineering
The NIH launched the HEAL Initiative in April 2018 to improve prevention and treatment strategies for opioid misuse and addiction and enhance pain management. The NIH HEAL Initiative aims to improve treatments for chronic pain, curb the rates of opioid use disorder and overdose and achieve long-term recovery from opioid addiction.
Carnegie Mellon’s award of more than $2 million is one of 375 grant awards across 41 states made by the National Institutes of Health in 2019 to apply scientific solutions to reverse the national opioid crisis.
“It’s clear that a multi-pronged scientific approach is needed to reduce the risks of opioids, accelerate development of effective non-opioid therapies for pain and provide more flexible and effective options for treating addiction to opioids,” said NIH Director Francis S. Collins, M.D., Ph.D., who launched the initiative in early 2018. “This unprecedented investment in the NIH HEAL Initiative demonstrates the commitment to reversing this devastating crisis.”
The research team, led by Principal Investigator Bin He, includes Carnegie Mellon investigators Matt Smith, associate professor of BME and the Neuroscience Institute; Kai Yu, research scientist in BME; and BME Ph.D. student Rachel Niu; as well as Kalpna Gupta, professor of medicine at the University of California, Irvine.
