The root of the matter
A team from the College of Engineering has used the natural architecture of the mangrove tree to unlock a better method of desalination.
For most plants, saltwater is essentially poison—yet the mangrove drinks it, lives in it, and thrives in it.
This rare ability to survive in such inhospitable conditions is what first led Professors Alan Russell and Phil LeDuc, along with their Ph.D. student Adam Wood, to study the plant. The Carnegie Mellon University researchers were hoping to determine exactly what part of the plant is responsible for removing the salt from saltwater, but their findings led them to much more.
Members of the mangrove family can be found emerging on stilt-like roots from the swampy coastal shorelines of the tropics and subtropics. In these regions, they face not only a toxically saline environment, but also oxygen-poor, submerged soil.
As they analyzed the inner workings of the plant, the researchers took note of its porous interior structure, consisting of a series of particularly wide, interconnected tubes collectively called the aerenchyma. This tissue allows for gas exchange between the exposed upper portions of the plant and the submerged, oxygen-deprived roots. It was while observing this structure, that Wood was struck by an idea.
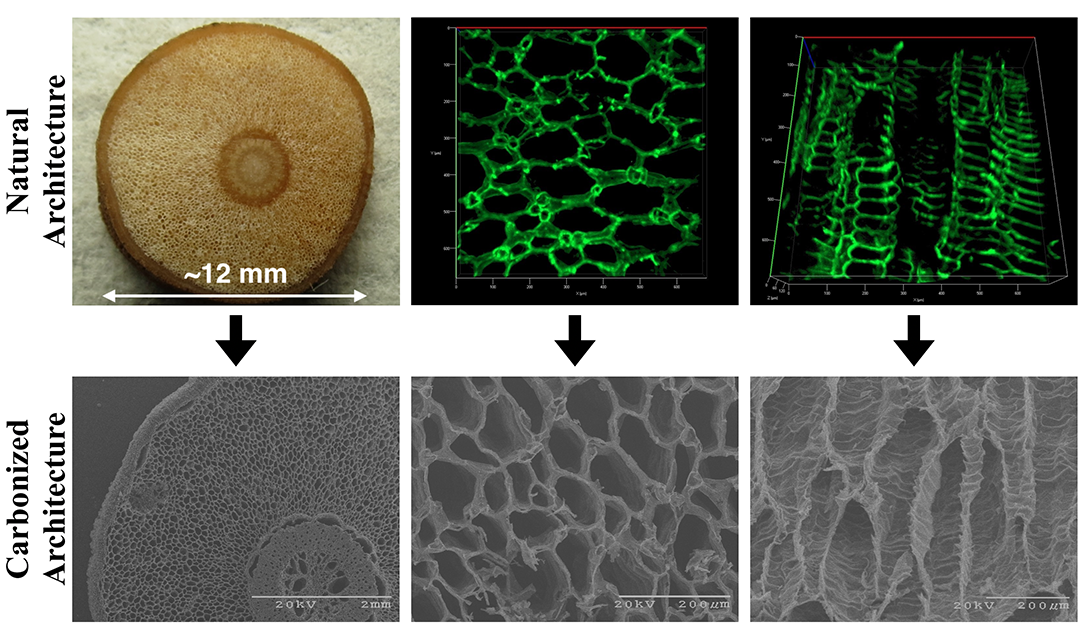
Source: College of Engineering
Magnified views of the mangrove aerenchyma tissue, before and after carbonization.
“Both Phil LeDuc and I have been interested and involved in tissue engineering using synthetic and natural materials for human health purposes,” said Russell, professor of chemical engineering. “He had the original idea that it would be interesting to look at plant materials for non-medical applications. We decided to co-advise Adam, and then Adam really took that general concept and came up with the idea to use mangrove roots as electrodes for desalination.”
While many in the past have carbonized, crushed, and packed together organic materials to create electrodes for desalination, Wood theorized that the aerenchyma of the mangrove root might make it a ready-made electrode. One simple heat treatment later—carbonizing the wood to make it conductive—and his suspicions were confirmed.
The team was able to create a desalination system with functioning electrodes using only intact, carbonized mangrove sections. He research suggests this technique could be more energy-efficient than other common technologies for desalinating brackish water.
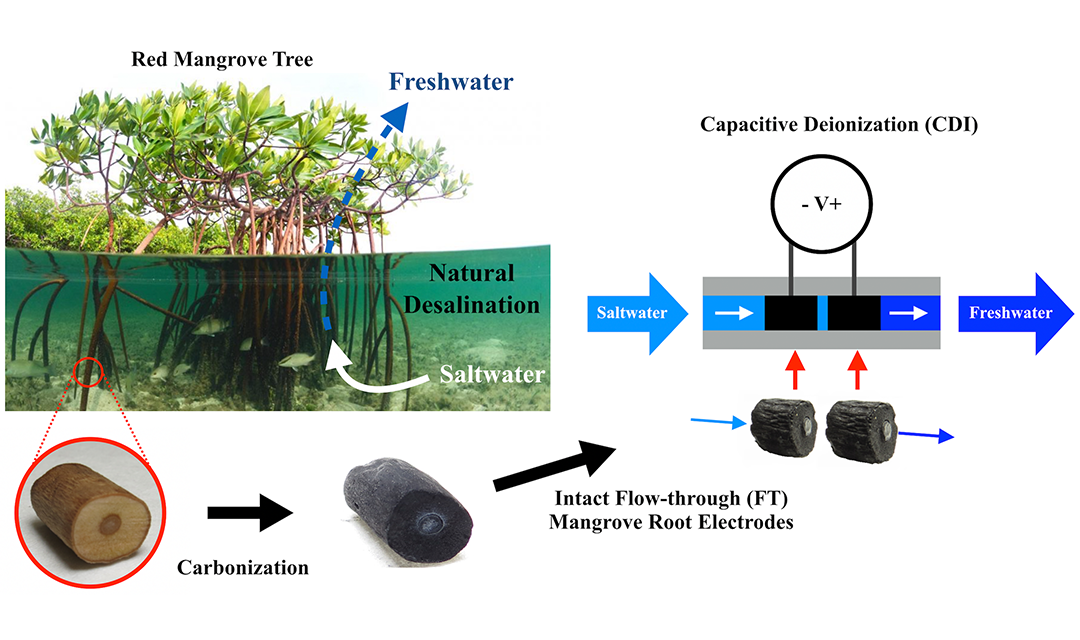
Source: College of Engineering
A diagram showing the assembly of the desalination system.
“In the United States, the vast majority of desalination plants are utilizing brackish water either at the surface or underground,” says Wood. “This is only becoming more important because of seawater intrusion. If you take more and more groundwater out, seawater actually starts to intrude.”
This overexploitation of natural resources threatens not only the drinking water supply, but water needed for animals, irrigation, and industry. The team’s research provides an easily created tool that could help mitigate this issue, as well as issues of water insecurity across the globe. It’s cheap, easy to prepare and assemble, and requires only a small power source. Their research suggests that some power can even be recovered from the system. Most importantly, the team believes their solution may not be limited solely to mangroves; the mangrove is not the only organic material with aerenchyma or a similarly porous structure.
“We're looking at other natural sources and natural architectures that are plant-derived where we don’t have to crush them, and that are perhaps even more scalable than using mangrove,” says Russell.
LeDuc, professor of mechanical engineering, adds, “It might be surprising to see chemical engineers, mechanical engineers, and material scientists coming together to study plants, but support for this kind of outside-the-box transdisciplinary work is what makes Carnegie Mellon so great.”
Co-authors of this research also included LeDuc Lab alumnus Kyle Justus, and the Department of Biomedical Engineering’s Assistant Professor Tzahi Cohen-Karni and Ph.D. student Raghav Garg.
