Forever chemicals, made simple
Civil and environmental engineering researchers provide a roadmap to simplify PFAS destruction in water resources using heterogeneous catalysis, a more sustainable and cost-effective approach.
They’re called “forever chemicals” for a reason. Per- and polyfluoroalkyl substances, better known as PFAS, exist everywhere from furniture and personal care products to drinking water, soils, and—consequently—our bodies. These synthetic chemicals are linked to serious health risks, including liver damage, reproductive issues, and certain cancers. But their signature carbon-fluorine bonds are one of the strongest known in organic chemistry, making PFAS, as the moniker suggests, notoriously difficult to destroy.
In response to growing concern, the U.S. Environmental Protection Agency recently enacted unprecedentedly strict limits on PFAS in drinking water—regulations that reflect the serious danger these chemicals pose. While current treatment methods like filtration and adsorption can help meet these standards, they are not destructive processes and generate PFAS waste streams that still require treatment.
To combat this, researchers at Carnegie Mellon University provide a roadmap toward a more effective, holistic, and sustainable process for removing PFAS from our drinking water. In a recent study, the team proposes using heterogeneous catalysis, coupled with pre- and post- treatment steps, to fully destroy PFAS compounds and eliminate them from our water sources. The overall goal is to fully destroy PFAS by breaking each and every one of their carbon-fluorine bonds with the least amount of reagents and energy.

Hosea Santiago-Cruz, Ph.D. student in the Department of Civil and Environmental Engineering, in the environmental engineering lab.
“Most research in this area develops treatments that can lower a specific PFAS concentration, but this typically produces other PFAS byproducts in the process,” said Hosea Santiago-Cruz, Ph.D. student in CMU’s Department of Civil and Environmental Engineering and co-first author of the study published in Nature Water. “Our holistic approach instead values how well the treatment breaks carbon-fluorine bonds, which we see as a better metric for comparing PFAS destruction approaches.”
High school chemistry classes teach that a catalyst is an agent that accelerates a chemical reaction without being consumed in the process. This makes catalytic processes—especially heterogeneous ones, where the catalyst is a different state of matter than the reactants—ideal for large-scale applications since the agent can be recovered, recycled, and reused.
Effective heterogeneous catalysts would be transformational for industrial-scale PFAS removal.
Greg Lowry, Professor, Civil and Environmental Engineering
“Effective heterogeneous catalysts would be transformational for industrial-scale PFAS removal since a solid is easily separated from contaminated water, in addition to the catalysis process being sustainable and readily scalable,” said Greg Lowry, professor of civil and environmental engineering.
One of the key obstacles to this approach, however, is the expansive variety within the PFAS chemical class. PFAS refers to a broad group of more than 15,000 substances, each with unique properties and increasingly complex structures that complicate removal efforts. Foam found in fire extinguishers used to fight fuel fires in airports, for example, can contain hundreds of PFAS compounds alone, many with unknown structures that present design and optimization challenges for catalyst development.
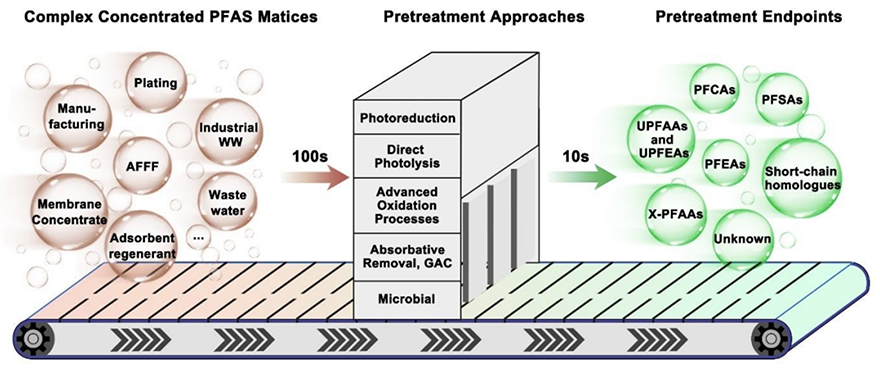
Processes proposed to simplify complex, unknown PFAS mixtures into fewer, more well-known compounds.
To simplify these complex PFAS mixtures, the authors propose a pre-treatment step using known homogeneous catalysis processes like oxidation or photoreduction.
“A homogeneous pre-treatment could reduce complex mixtures, like in the case of firefighting foams, from hundreds of PFAS, many of which are unknown, to just tens of PFAS molecules that are more manageable to destroy,” explained Lowry. “The overall goal of pre-treatment is to streamline the development of the heterogeneous catalysis process and lower analytical and energy costs.”
A homogeneous pre-treatment could reduce complex mixtures from hundreds of PFAS to just tens of PFAS molecules that are more manageable to destroy.
Grey Lowry, Professor, Civil and Environmental Engineering
While there are catalysts that can break down a few known PFAS chemicals in contaminated water, more work lies ahead to identify catalysts for a larger range of PFAS compounds for large-scale use. Lowry and his team suggest implementing molecular modeling and machine learning technologies to enhance the development process, identifying opportunities for discerning PFAS traits, grouping similar compounds, and overall increasing predictive understanding in the field to treat real PFAS mixtures.
“Robust, cost- and energy-efficient methods for removing PFAS from drinking water and industrial waste streams is going to require research and technological advancements, and this approach is a step in the right direction,” he said.
