Insights on limits of non-invasive deep-brain stimulation
Carnegie Mellon University researchers unveil groundbreaking insights into the mechanisms and limitations of temporal interference stimulation for non-invasive neurostimulation.
Being able to noninvasively stimulate the deep brain without stimulating shallow regions is a key goal of non-invasive neurostimulation. A study published in Nature Communications Biology by Carnegie Mellon University researchers provides a breakthrough in understanding neural mechanisms of a recent neurostimulation technique called temporal interference (TI) stimulation that is thought to be capable of reaching this goal. The research obtains new understanding of cell-specific effects of TI stimulation in the brain, which has profound implications on the use of TI stimulation for treatment of brain conditions.
Transcranial electrical stimulation (TES) is commonly used for stimulating targeted brain regions in neuroscientific studies and clinical treatments. Temporal interference stimulation is a variation of TES that combines different electrical frequencies in order to attempt to obtain deep stimulation without stimulating the shallow brain. The study obtains evidence suggesting that parvalbumin (PV) neurons are activated with TI stimulation in both the deep and shallow brain. However, in the shallow brain, inhibitory effects of PV neurons are strong, preventing excitatory pyramidal (Pyr) neurons from activating. TI stimulation causes high activation of PV neurons in shallow brain regions and lower activation in deep regions, possibly allowing Pyr neurons to fire in the deep regions. This helps explain why Pyr neurons in targeted deep brain regions activate while those in off-target shallow regions do not.
The work exposes limitations and side effects of TI stimulation, but it also might inspire alternative approaches to the problem of non-invasive deep brain stimulation.
Pulkit Grover, Professor, Electrical and Computer Engineering
Sara Caldas Martinez, a Ph.D. student studying biology, made a key observation. Through measuring membrane potentials of cells in response to TI stimulation, Martinez found that when Pyr neurons are disconnected from the rest of the neural network, they no longer respond to TI stimulation. This finding is significant because it corrects a widely held belief that neurons respond individually to TI stimulation. The study shows that TI stimulation is fundamentally a network phenomenon.
These discoveries are significant because improved understanding and control of neural mechanics can lead to improved treatment for conditions like depression, PTSD, OCD, addiction, and substance abuse disorder.
“The insight that temporal interference stimulation is primarily a result from the network, rather than a single cell’s intrinsic properties, shifts the current mechanistic understanding and its potential applications,” said Martinez. “It also opens the door to new possibilities, especially for reducing excitability, which is a key feature of some neurological disorders like certain forms of autism.”
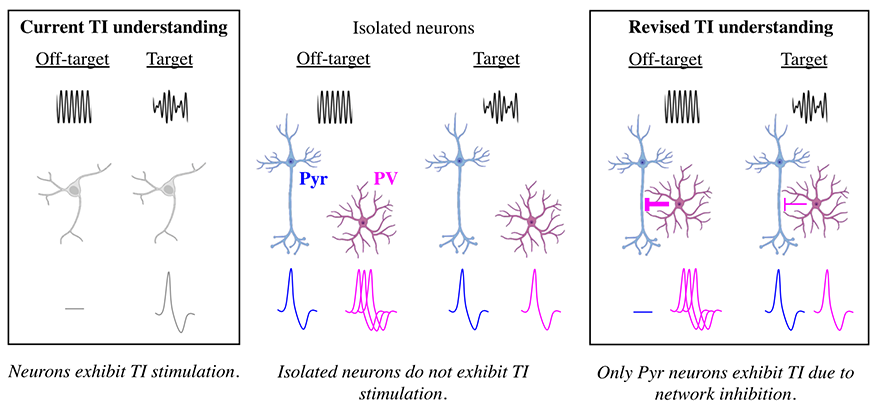
TI stimulation causes high activation of PV neurons in shallow brain regions and lower activation in deep regions, possibly allowing Pyr neurons to fire in the deep regions. The study shows that TI stimulation is fundamentally a network phenomenon.
Unlike other procedures, TI stimulation is non-invasive. It does not require electrodes to be inserted into the brain. Instead, it uses an external device to send electrical signals into the brain. Pulkit Grover, a professor of electrical and computer engineering and member of the Neuroscience Institute, said invasive procedures have historically had favorable results for treating illness, so achieving similar results through more widely adoptable non-invasive TI stimulation would be desirable.
“You can do really cool things, including possibly treating conditions by improving focality, selectivity, and specificity, and by tailoring it to the patient,” said Grover. “However, the limitations are substantial.”
The study points to TI stimulation’s limitations and potential side effects, including a high propensity for shallow brain stimulation.
This study was an interdisciplinary collaboration between Grover and Alison Barth, a professor of life sciences and member of the Neuroscience Institute.
Now we can begin the hard work of testing clinical applications for brain disorders like epilepsy, chronic pain, and even some forms of depression.
Allison Barth, Professor of the Life Sciences, Mellon College of Science
“Non-invasive brain stimulation has a lot of promise for inducing brain plasticity, but without a mechanistic understanding of how it works, it's been hard to test it. This study establishes a neural basis for its effects,” Barth explained. “It is really exciting to see that we can inhibit neural activity using this technique. Now we can begin the hard work of testing clinical applications for brain disorders like epilepsy, chronic pain, and even some forms of depression.”
“Digging into the mechanisms of why something works is important. I learned I was wrong about why TI stimulation was working and how it could be used,” said Grover. “The work exposes limitations and side effects of TI stimulation, but it also might inspire alternative approaches to the problem of non-invasive deep brain stimulation.”
More information: Caldas-Martinez, S., Goswami, C., Forssell, M., Cao, J., Barth, A. L., & Grover, P. (2024). Cell-specific effects of temporal interference stimulation on cortical function. Nature Communications Biology, 7(1), 1076.
