Detecting brain tsunamis
Researchers from Carnegie Mellon University, the University of Pittsburgh, and the University of Cincinnati have combined their expertise in engineering and medicine to create a noninvasive method for detecting worsening brain injuries before they happen. This advancement could reshape neurocritical care.
A collaboration between researchers in engineering and medicine from Carnegie Mellon University, the University of Pittsburgh, and the University of Cincinnati has resulted in the first noninvasive method for detecting worsening brain injuries before they happen, a breakthrough with potential to reshape neurocritical care.
This approach is the subject of research by Pulkit Grover and collaborators that was recently published in Nature Communications Medicine
Grover, a professor of electrical and computer engineering and the Neuroscience Institute at CMU, leads the For All Lab, a neuroengineering lab that works to develop novel diagnosis and treatment tools that span theoretical, computational, and hardware approaches, and are accessible to the broadest possible population. He explains that electroencephalography (EEG) is a routine procedure with no risks or side effects that is widely used in clinics.
“The patient is asked to sit in a chair or lie in a bed while a technician installs electrodes on their scalp to record brain signals,” said Grover. “Modern EEG is also portable, so people can walk around and carry on in their daily lives while signals are being recorded.”
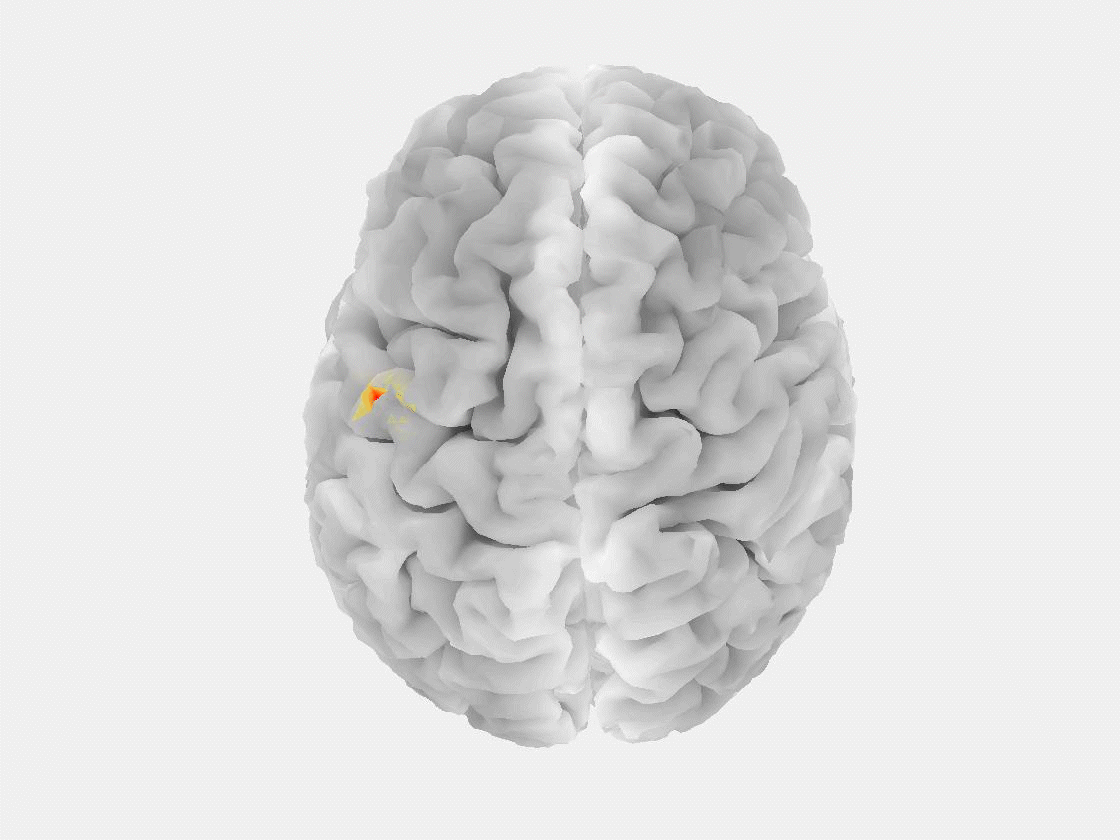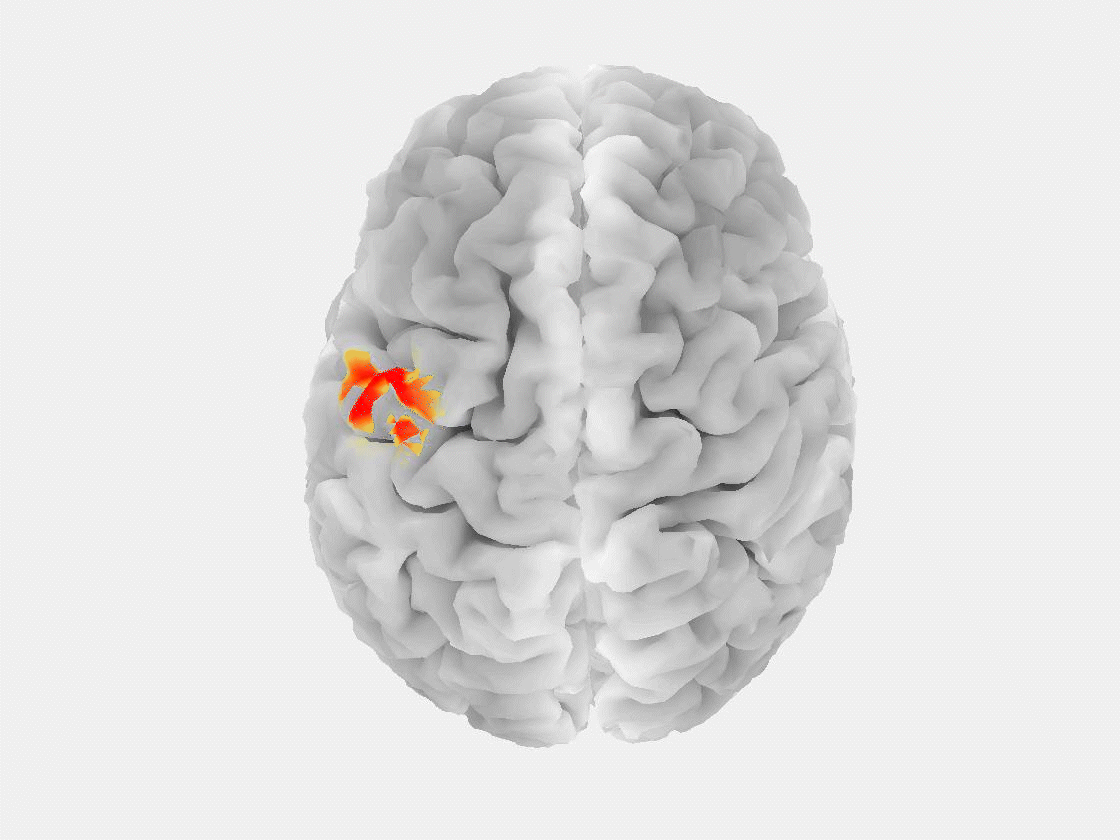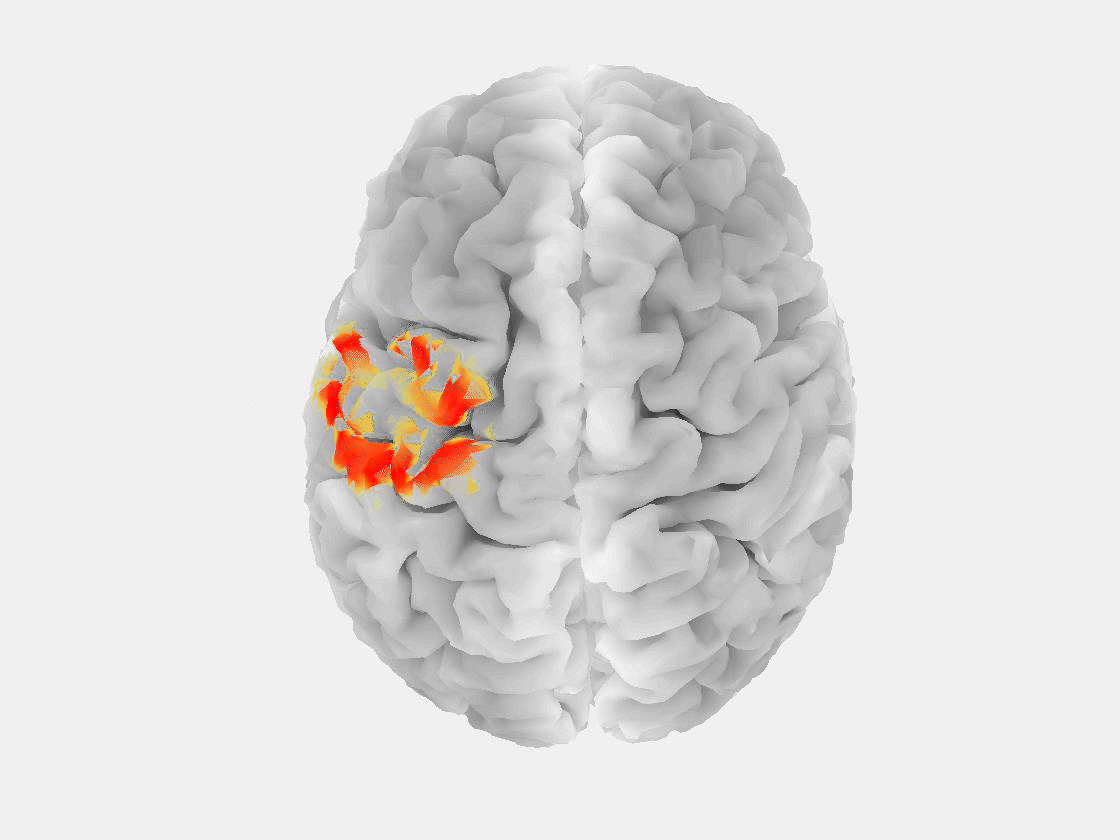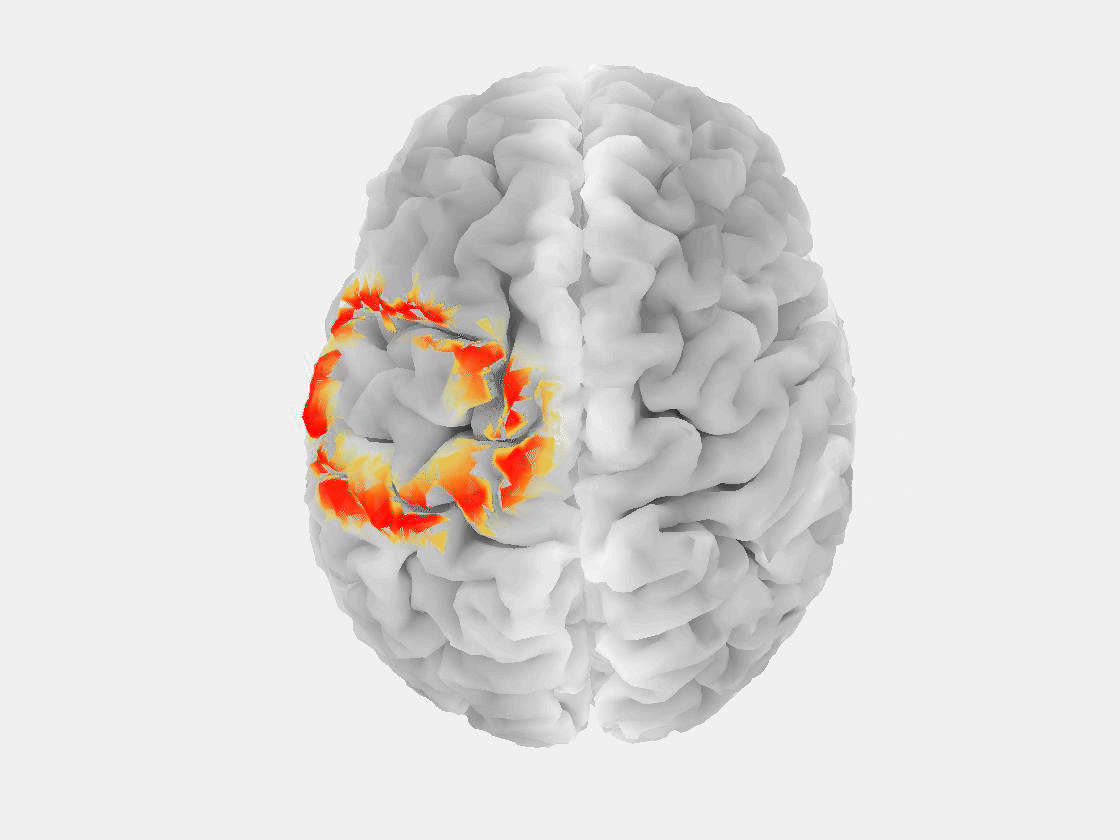
This research marks the first time that EEG, or any non-invasive measurement technique, has been used to reliably detect a “brain tsunami,” a colloquial term for a spreading depolarization, which represents a wave of reduced activity that moves across the surface of the brain at a rate of a few millimeters per minute.
Besides spreading depolarization, there are no other known biomarkers that help you predict worsening brain injuries before they happen.
Pulkit Grover, Professor, Electrical and Computer Engineering
“Spreading depolarization is known to be predictive of worsening brain injuries, so detecting it enables use of corrective treatment,” Grover said. “There are no other known biomarkers that help you predict worsening brain injuries before they happen.”
The lead author on the work is Alireza Chamanzar, a postdoctoral research associate in electrical and computer engineering, who focused on the problem of detecting spreading depolarizations during his Ph.D. The team’s earlier work, on localizing neural silences, provided the foundations for this effort.
“Traumatic brain injury is a heterogeneous disease, and there is no objective diagnostic and treatment measure available to clinicians,” Chamanzar said. “Continuous monitoring of spreading depolarization, as a reliable biomarker and potential therapeutic target, leads to precision medicine and a reduction in secondary injury.”

Source: Chuck Noll Foundation
Pulkit Grover is seen with a short haircut as he participates in neuroscience experiments.
Researchers have tried for decades to non-invasively detect spreading depolarization, recognizing its potential for improving patient care. One of the challenges is how to detect these traveling waves while dealing with a complex brain structure with folds. Having an interdisciplinary group work on this research was crucial so that each collaborator could use their respective expertise in engineering, medicine, or clinical research to make this lofty goal reachable.

Source: Alireza Chamanzar
Alireza Chamanzar, a Ph.D. graduate of the Electrical and Computer Engineering Department, previously worked with Grover on research that served as the foundation for this study.
The starting point was an algorithm called WAVEFRONT, developed originally in 2019 by Chamanzar, who was then a Ph.D. student in Grover’s lab. The first iteration of the model was trained with simulated data to measure electrode signals, but simulations are only so accurate. Real-world data was needed to take things to the next level. WAVEFRONT was retrained using spreading depolarization data gathered from 12 patients via intracranial recording and electroencephalography, sourced from a study at the University of Cincinnati. WAVEFRONT validated this information with an impressive level of accuracy.
The team is actively working on improving the accuracy of their algorithm even further, especially to detect subtle signals from migraines and concussions. The end goal is to provide a new tool for clinical use that personalizes neurocritical care.
“For too long, patients have been grouped crudely into mild, moderate, and severe traumatic brain injury categories. This will help better stratify patients, and ultimately, provide better, more personalized care for this debilitating condition that affects millions every year,” said Grover.
The work was supported by the Chuck Noll Foundation for Brain Injury Research, the Center for Machine Learning and Health, and the National Science Foundation. The work was co-authored by Jed Hartings, professor of neurosurgery at the University of Cincinnati; Jonathan Elmer, associate professor of emergency medicine, critical care medicine, and neurology at the University of Pittsburgh; and Lori Shutter, professor of neurology at the University of Pittsburgh.