Advancing dynamic brain imaging with AI
New research from Carnegie Mellon University’s Bin He introduces a novel, AI-based dynamic brain imaging technology alternative which could map out rapidly changing electrical activity in the brain with high speed, high resolution, and low cost.
MRI, electroencephalography (EEG) and magnetoencephalography have long served as the tools to study brain activity, but new research from Carnegie Mellon University introduces a novel, AI-based dynamic brain imaging technology which could map out rapidly changing electrical activity in the brain with high speed, high resolution, and low cost. The advancement comes on the heels of more than 30 years of research that Bin He has undertaken, focused on ways to improve non-invasive dynamic brain imaging technology.
Brain electrical activity is distributed over the three-dimensional brain and rapidly changes over time. Many efforts have been made to image brain function and dysfunction, and each method bears pros and cons. For example, MRI has commonly been used to study brain activity, but is not fast enough to capture brain dynamics. EEG is a favorable alternative to MRI technology; however, its less-than-optimal spatial resolution has been a major hindrance in its wide utility for imaging.
Electrophysiological source imaging has also been pursued, in which scalp EEG recordings are translated back to the brain using signal processing and machine learning to reconstruct dynamic pictures of brain activity over time. While EEG source imaging is generally cheaper and faster, specific training and expertise is needed for users to select and tune parameters for every recording. In new published work, He and his group introduce a first of its kind AI-based dynamic brain imaging methodology, that has the potential of imaging dynamics of neural circuits with precision and speed.
“As part of a decades-long effort to develop innovative, non-invasive functional neuroimaging solutions, I have been working on a dynamic brain imaging technology that can provide precision, be effective and easy to use, and better serve clinicians and researchers,” said Bin He, professor of biomedical engineering at Carnegie Mellon University.
As part of a decades-long effort to develop innovative, non-invasive functional neuroimaging solutions, I have been working on a dynamic brain imaging technology that can provide precision, be effective and easy to use, and better serve clinicians and researchers.
Bin He, Professor, Biomedical Engineering
He continues, “Our group is the first to reach the goal by introducing AI and multi-scale brain models. Using biophysically inspired neural networks, we are innovating this deep learning approach to train a neural network that can precisely translate scalp EEG signals back to neural circuit activity in the brain without human intervention.”
In He’s study, which was recently published in Proceedings of the National Academy of Sciences (PNAS), the performance of this new approach was evaluated by imaging sensory and cognitive brain responses in 20 healthy human subjects. It was also rigorously validated in identifying epileptogenic tissue in a cohort of 20 drug-resistant epilepsy patients by comparing AI based noninvasive imaging results with invasive measurements and surgical resection outcomes.
Results wise, the novel AI approach outperformed conventional source imaging methods when precision and computational efficiency are considered.
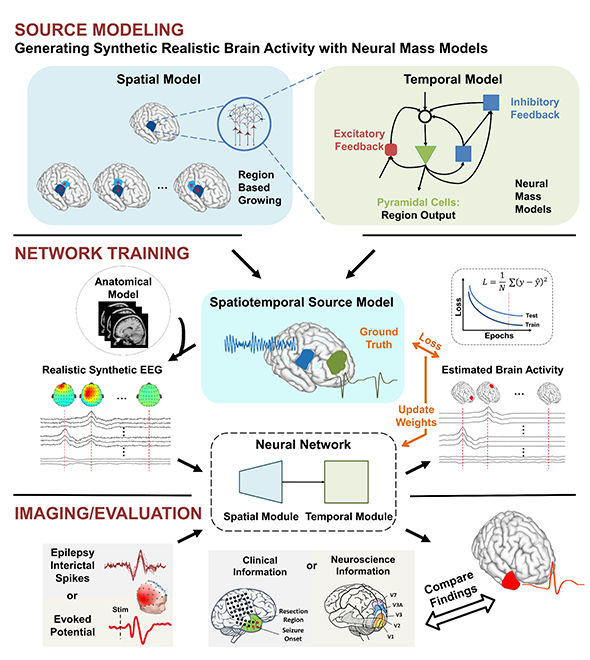
Source: College of Engineering
Concept of the novel deep learning-based source imaging framework.
“With this new approach, you only need a centralized location to perform brain modeling and training deep neural network,” explained He. “After collecting data in a clinical or research setting, clinicians and researchers could remotely submit the data to the centralized well trained deep neural networks and quickly receive accurate analysis results. This technology could speed up diagnosis and assist neurologists and neurosurgeons for better and faster surgical planning.”
As a next step, the group plans to conduct larger clinical trials in efforts to bring the research closer to clinical implementation.
“The goal is for efficient and effective dynamic brain imaging with simple operation and low cost,” explained He. “This AI-based brain source imaging technology makes it possible.”
This work was supported in part by the National Institute of Neurological Disorders and Stroke, National Institute of Biomedical Imaging and Bioengineering, National Center for Complementary and Integrative Health, National Institute of Mental Health of the National Institutes of Health (NIH), NIH BRAIN Initiative and NIH HEAL Initiative. The work was also partially supported by the Pittsburgh Health Data Alliance and used facility at the Pittsburgh Supercomputing Center. Other collaborators on the PNAS paper include the first author Rui Sun, a BME Ph.D. student in He’s lab; Abbas Sohrabpour, a former BME postdoctoral associate in He’s lab; and clinical collaborator Gregory Worrell of the Mayo Clinic.
