Unlocking richer intracellular recordings
A forward-thinking group of researchers from Carnegie Mellon University and Istituto Italiano di Tecnologia has identified a flexible, low-cost, and biocompatible platform for enabling richer intracellular recordings.
Behind every heartbeat and brain signal is a massive orchestra of electrical activity. While current electrophysiology observation techniques have been mostly limited to extracellular recordings, a forward-thinking group of researchers from Carnegie Mellon University and Istituto Italiano di Tecnologia has identified a flexible, low-cost, and biocompatible platform for enabling richer intracellular recordings.
The group’s unique “across the ocean” partnership started two years ago at the Bioelectronics Winter School (BioEl) with libations and a bar napkin sketch. It has evolved into research published today in Science Advances, detailing a novel microelectrode platform that leverages three-dimensional fuzzy graphene (3DFG) to enable richer intracellular recordings of cardiac action potentials with high signal to noise ratio. This advancement could revolutionize ongoing research related to neurodegenerative and cardiac diseases, as well as the development of new therapeutic strategies.
A key leader in this work, Tzahi Cohen-Karni, associate professor of biomedical engineering and materials science and engineering, has studied the properties, effects, and potential applications of graphene throughout his entire career. Now, he is taking a collaborative step in a different direction, using a vertically-grown orientation of the extraordinary carbon-based material (3DFG) to access the intracellular compartment of the cell and record intracellular electrical activity.
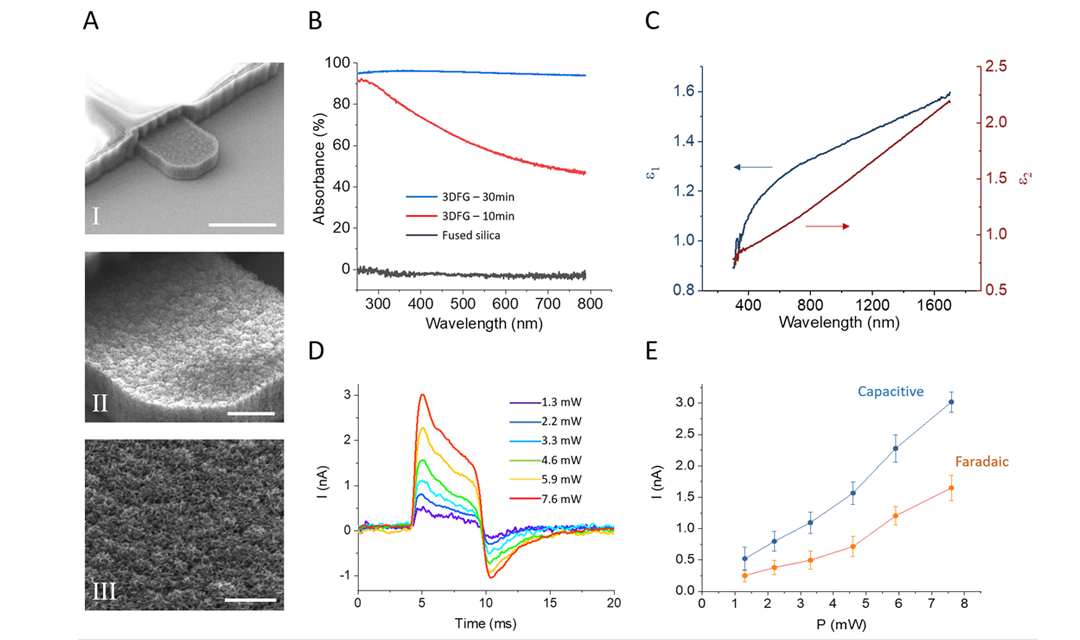
Source: College of Engineering
SEM images provide a closer look at 3DFG electrodes.
Due to its unique electrical properties, graphene stands out as a promising candidate for carbon-based biosensing devices. Recent studies have shown the successful deployment of graphene biosensors for monitoring the electrical activity of cardiomyocytes, or heart cells, outside of the cells, or in other words, extracellular recordings of action potentials. Intracellular recordings, on the other hand, have remained limited due to ineffective tools…until now.
“Our aim is to record the whole orchestra—to see all the ionic currents that cross the cell membrane—not just the subset of the orchestra shown by extracellular recordings,” explains Cohen-Karni. “Adding the dynamic dimension of intracellular recordings is fundamentally important for drug screening and toxicity assay, but this is just one important aspect of our work.”
“The rest is the technology advancement,” Cohen-Karni continues. “3DFG is cheap, flexible, and an all-carbon platform; no metals involved. We can generate wafer-sized electrodes of this material to enable multi-site intracellular recordings in a matter of seconds, which is a significant enhancement from an existing tool, like a patch clamp, which requires hours of time and expertise.”
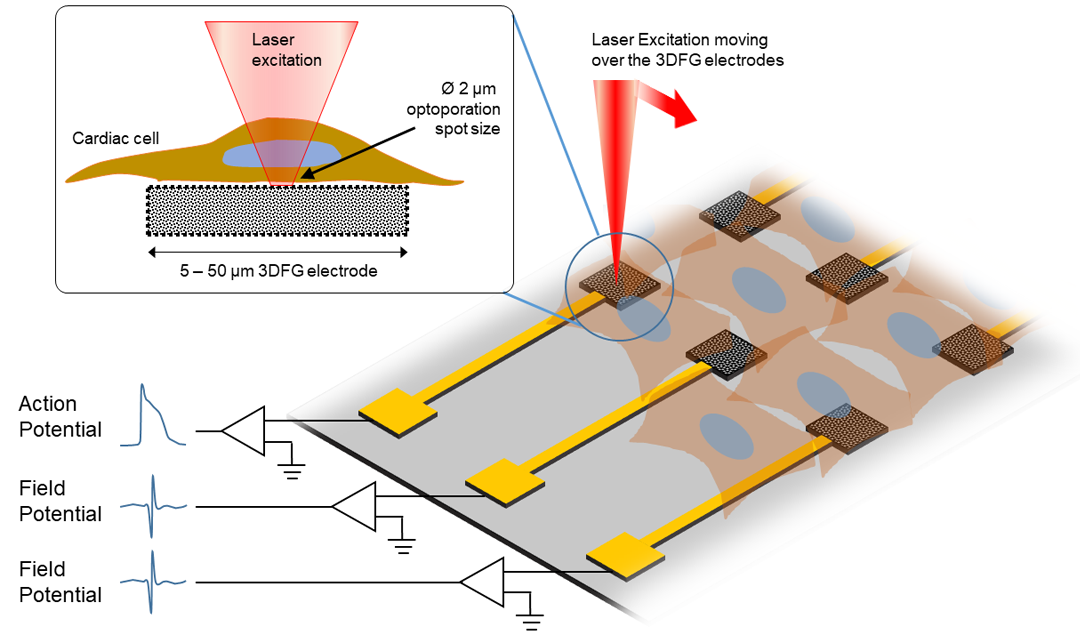
Source: College of Engineering
This sketch displays the experimental procedure of ultra-fast laser moving over the 3DFG electrodes.
So, how does it work? Leveraging a technique developed by Michele Dipalo and Francesco De Angelis, researchers at Istituto Italiano di Tecnologia, an ultra-fast laser is used to access the cell membrane. By shining short pulses of laser onto the 3DFG electrode, an area of the cell membrane becomes porous in a way, allowing for electrical activity within the cell be recorded. Then, the cardiomyocytes, are cultured to further investigate interactions between the cells.
Interestingly, 3DFG is black and absorbs most of the light, resulting in unique optical properties. Combined with its foam-like structure and enormous exposed surface area, 3DFG has many desirable traits that are needed to make small biosensors.
“We have developed a smarter electrode; an electrode that allows us better access,” emphasizes Cohen-Karni. “The biggest advantage from my end is that we can have access to this signal richness, to be able to look into processes of intracellular importance. Having a tool like this will revolutionize the way we can investigate effects of therapeutics on terminal organs, such as the heart.”
We have developed a smarter electrode; an electrode that allows us better access.
Tzahi Cohen-Karni, Associate Professor, Biomedical Engineering, Materials Science and Engineering
Additional contributions to the project were made by Adam Feinberg, professor of biomedical engineering and materials science and engineering, and Sheng Shen, professor of mechanical engineering. Feinberg and his lab team assisted with pieces of the cardiomyocyte research done at CMU, while Shen’s lab team focused on the characterization of graphene’s optical properties.
As this work moves forward, the team plans to apply its learnings in large-scale cell/tissue interfaces, to better understand tissue development and toxicity of chemical compounds (e.g. drug toxicity).
Other team members involved in the research included Biomedical Engineering Ph.D. students Sahil Rastogi and Jacqueline Bliley; Materials Science and Engineering Ph.D. student Raghav Garg; Mechanical Engineering Ph.D. student Ramesh Shrestha; and the Istituto Italiano di Tecnologia’s Francesco de Angelis, Michele Dipalo, Giovanni Melle, Giuseppina Iachetta, Laura Matino, Francesca Santoro, and Andrea Barbaglia.
