A glowing biosensor for high-throughput drug screening
Researchers develop a bioluminescent sensor to test protein activation, speeding up a lengthy step in drug discovery for neurodegenerative diseases like Alzheimer’s Disease.
Drug discovery can be a long and complex process. Medicines for neurodegenerative diseases like Alzheimer’s Disease are among the most expensive to develop, as animal model results have not proven to be predictive of efficacy in humans. Scientists usually have to screen many biological targets before identifying a single potential new drug.
Researchers at Carnegie Mellon University are developing a platform to enable high-throughput drug screening. Their work is part of efforts to optimize each piece of the drug discovery process, with real impacts in the race to treat patients.
Anne Skaja Robinson investigates G-Protein Coupled Receptors (GPCRs), proteins that reside at the cell’s surface. They are the target of many small-molecule drugs, including therapies for diabetes, allergies, and cancer. The Robinson Lab is focused on the role of these transmembrane proteins and their downstream cellular responses. One side of a GPCR faces into the cell, where it’s associated with a G-protein. The other side of a GPCR is outside the cell, where a drug can bind; thus, they serve as sensors for a cell’s environment.
“There’s a lot of untapped therapeutic potential,” says Sarah Sonbati. There are 800 known GPCRs, yet current drugs target less than 15% of those.
The gap in disease treatments exists because scientists don’t yet know what binds to some GPCRs. Identifying small molecules to activate these orphan GPCRs (oGPCRs) is one path to possible new drugs.

Source: Sarah Sonbati
Inspired by the transient light emitted by fireflies, the Robinson Lab’s biosensor can detect the activation of G-protein coupled receptors.
Alzheimer’s Disease is of particular interest because scientists know that there is an upregulation, or an increase, of specific GPCRs in people with the disease. There’s also no cure yet. “What if we start looking at the root cause of Alzheimer’s Disease, and we try to target that?” asks Sonbati, a chemical engineering Ph.D. student. “The answer might be in GPCRs and understanding how a GPCR is activated.”
When a drug combines with the GPCR on the outside of the cell membrane, the G-protein inside the cell dissociates. Sonbati leveraged that change in association to create a biosensor that uses bioluminescence to detect the coupling of GPCRs and G-protein.
“Our platform enables more specific detection of the protein activation itself, and in a cellular context,” says Robinson, professor of chemical engineering. Robinson and Sonbati developed their cell-based assay from an existing system that uses an enzyme specifically designed to provide transient luminescence, based on the light emitted by fireflies. The enzyme luciferase is split into a small bit and a large bit, each attached to a protein. When the two proteins are interacting, the luciferase bits are close enough to reconstitute function and glow.
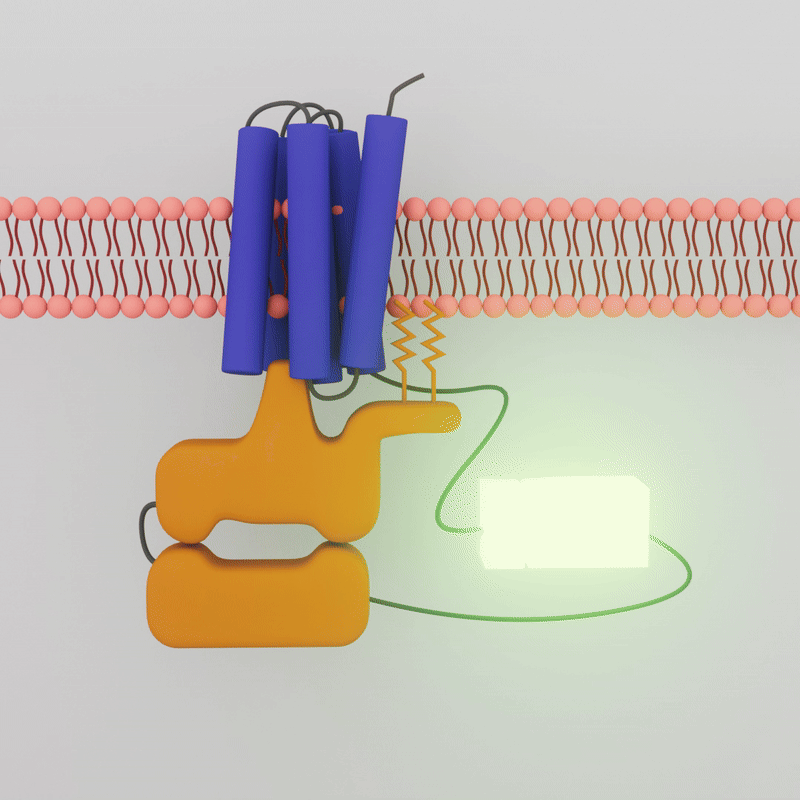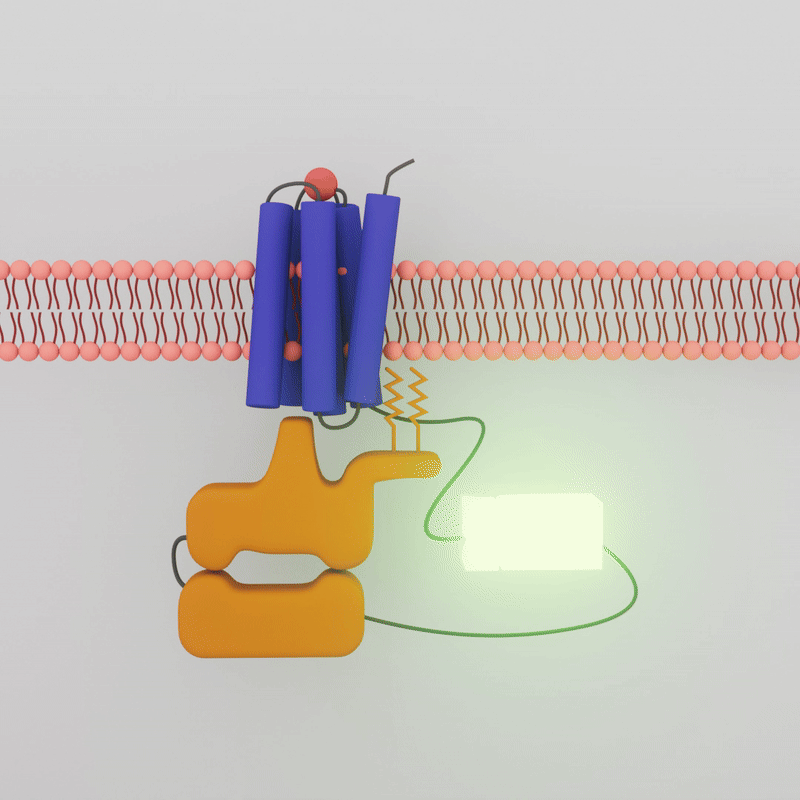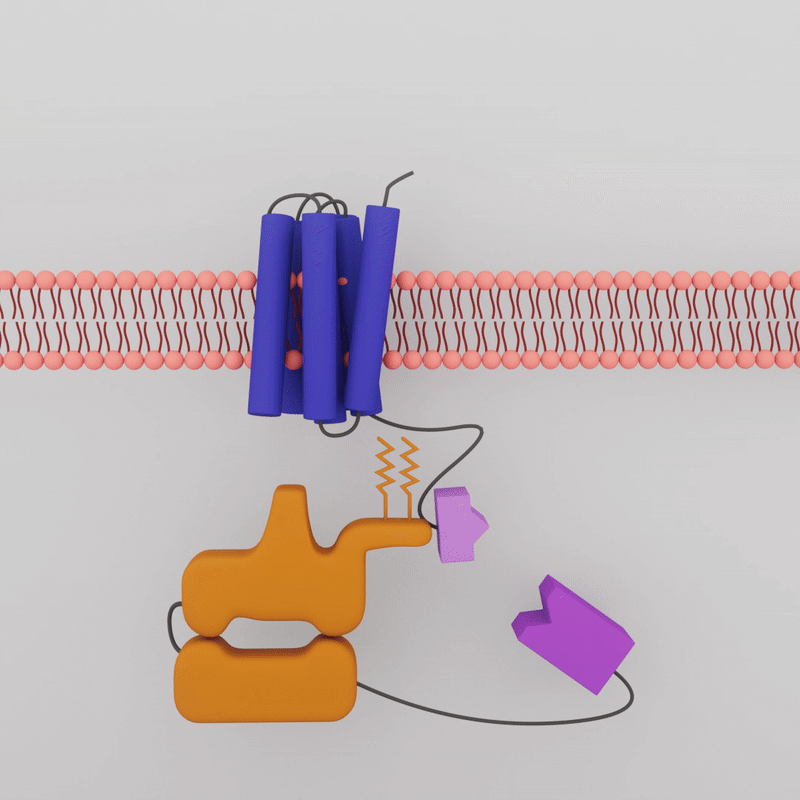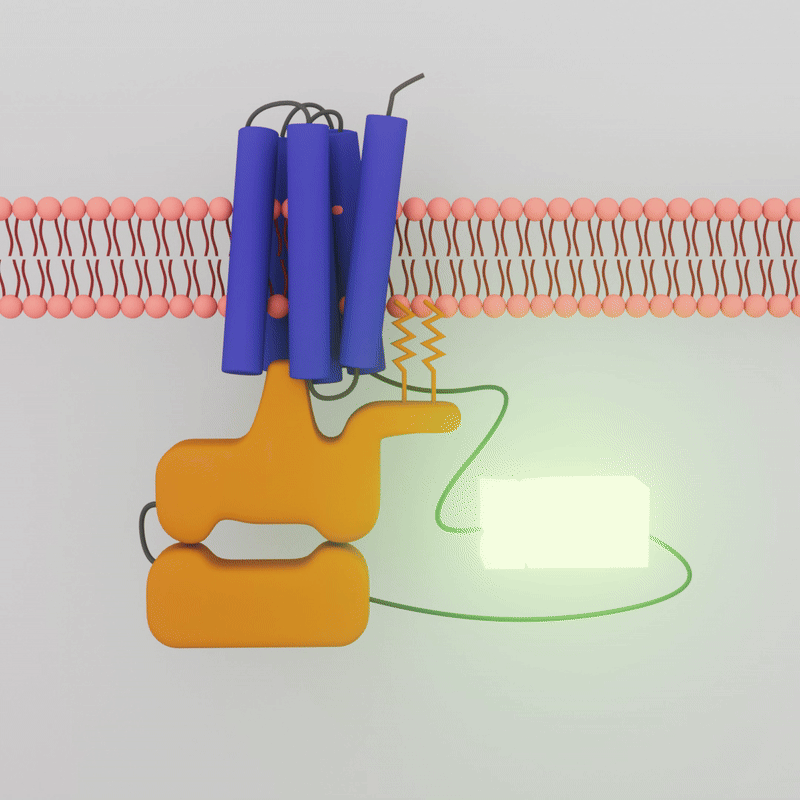
The transient luminescence of the biosensor depends on whether the G-Protein Coupled Receptor and the G-Protein are interacting. When a drug molecule combines with the GPCR on the outside of the cell membrane, the G-protein inside the cell dissociates, the two attached enzyme bits separate, and the glow is lost.
Sonbati optimized the approach using a GPCR that the Robinson Lab has worked with extensively, the adenosine A2A receptor. “We needed to understand what the data looked like in a well-characterized system, understand what it tells us about the interactions in the cell, before entering that unknown space of orphan GPCRs,” says Sonbati.
The drugs that activate A2A are known. Sonbati looked at two classes of drugs: agonists, which upregulate receptor activity; and inverse agonists, which downregulate receptor activity. Initially, the A2A is already bound, or “pre-coupled,” with the G-protein, creating bioluminescence even in a resting state in the cell. When an agonist is added, the GPCR and G-protein should dissociate, and the sensor should no longer show luminescence. Sonbati’s results confirmed this behavior from the control protein.
When an inverse agonist is added, the G-protein is recruited back to the GPCR, and luminescence increases again. “These results helped us understand that we're not always expecting to see a decrease in luminescence. We're looking for changes compared to the initial state, which will give us more information about our sensor,” says Sonbati.
No one has been able to look at these protein receptors before in quite this way.
Sarah Sonbati, Ph.D. student, Chemical Engineering
After optimizing for cell type, density, and transfection methods, Sonbati successfully applied the platform to two orphan GPCRs that are upregulated in Alzheimer’s Disease. “No one has been able to look at them before in quite this way,” she says.
The sensor also confirmed that both of the orphan GPCRs are pre-coupled to the G-protein, like A2A is. They don’t require another molecule to interact or activate functions in the cell. “That means everything we learned about luminescence with A2A can be applied in this space,” says Sonbati.
The Robinson Lab is now testing a 700-drug library from the National Institutes of Health (NIH) to see if any activate the orphan GPCRs they are working with. Sonbati has also designed constructs to switch their platform from mammalian cells to yeast. Yeast grows faster and is more robust, enabling more experiments and faster results.
Robinson and Sonbati’s platform is a powerful tool to test and understand GPCR activation. It is quicker and less expensive than traditional methods.
“Our vision is to apply this more generally for high-throughput drug screening,” says Sonbati. “Picture a well plate with our sensor in each small well. You add a different potential drug to each well. All the wells start glowing, meaning the proteins are interacting, except for one. The drug in that well is the one you want to look at further.”
By combining these tools with emerging machine learning technologies, Robinson and Sonbati hope to open new avenues for drug targets for Alzheimer's Disease and other diseases that have been less tractable for treatment.
Pictured, top: A typical G-protein coupled receptor (GPCR) at the cell’s surface
