Real-time sensors to inform better wound care
Wound assessment can be challenging due to its subjective nature, but a new sensor array quantifies biomarkers and has potential to offer real time measurements that could improve the healing process.
Wound assessment by medical professionals relies largely on visual inspection, which can be challenging due to its subjective nature. Nitric oxide (NO) plays an important role in healing, as it is produced by immune cells during inflammation and acts as a key signal to coordinate recovery. However, measuring NO in wounds is difficult for several reasons. It has a short lifespan, breaking down in a matter of seconds, so detection tools must work almost instantly to discover NO before it disappears. Additionally, NO exists in wounds at extremely low concentrations and most sensors aren’t sensitive enough to detect these trace levels. Fluid found in wounds can also contain bacteria and other substances that can skew measurements.
A new technology, the multiplexed, electrochemical, real-time, localized, inflammation-tracking nitric oxide (MERLIN) sensor array, has been developed by the Cohen-Karni research group to overcome these challenges. The chemical-electrical sensor, similar to a glucose monitor, quantifies biomarkers related to wound healing. Using flexible materials and multiple detection points to map NO levels across wounds, the technology will offer real time measurements that could improve the healing process.
Recent work published in Science Advances indicates promising results from in vivo testing on rat skin wounds. Over the course of seven days of testing, NO levels peaked on day three, reflecting the inflammation phase of wound healing, and falling in line with activity reported in previous studies. Following the promising outcomes from these tests, the sensor is set to enter human trials within the next month at University of Pittsburgh Medical Center (UPMC).
“The sensors will help clinicians assess the state of a wound by directly measuring the levels of nitric oxide, a biomarker for the immune system response to the wound,” said Tzahi Cohen-Karni, a professor of biomedical engineering and materials science and engineering.
The sensors were developed as a result of breakthroughs in wound care under a DARPA funded project in the Bioelectronics for Tissue Regeneration program. The latest work will provide support for diagnostic tools in addition to therapeutic interventions.
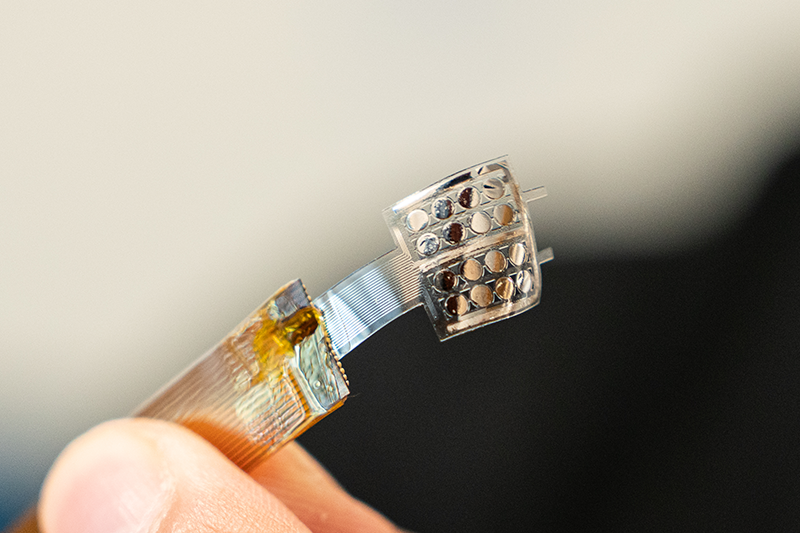
The flexible device will be able to conform to a wound’s topology to accurately measure nitric oxide levels.
In testing these sensors, the research group, which included collaborators from Carnegie Mellon University, University of Pittsburgh, and Lake Erie College of Osteopathic Medicine, have considered the importance of flexibility, size, and speed in their prototype. As a result, the array can be fabricated as a modular component and integrated into a biohybrid electronic platform.
“While the current iteration of the device is intended to be deployed in a clinical setting by a physician, future improvements in the technology could be incorporated into a patch that a patient could use at home and clinicians could access to the data remotely to monitor their patients,” said Cohen-Karni.
As this sensor array is further developed, the research group will seek to monitor NO levels beyond seven days in order to capture its role in healing and preventing scarring in chronic and delayed wound healing.
