The force to shape an organ
Novel biosensor reveals the mechanobiological forces that shape organ development and biological phenomena like hypertension.
Biomedical Engineering and Materials Science and Engineering Professor Adam Feinberg along with postdoctoral fellow Dan Shiwarski and graduate student Joshua Tashman have created a novel biosensor that reveals the mechanobiological forces that shape organ development. These little-understood forces are of increasing interest to medicine and research and the team’s findings offer important insights vital for understanding disease, as well as the future of bioengineered organs.

Source: Carnegie Mellon University
Timelapse showing each step in using the NMBS to map mechanobiological forces.
In this instance, a mechanobiological force refers to the change in shape and size of a tissue resulting from mechanical forces that cells exert on surrounding cells and the extracellular environment. While mechanical forces are an integral component of tissue development, measuring these forces in three dimensions—let alone in living tissue—is extremely complex. The incorporation of physiologic forces, especially those that drive proper development, has been largely overlooked in bioengineered tissue and organ research due to a lack of quantitative information. If researchers could directly measure these forces and the resulting mechanical strains within living tissue during development it could lead to both a critical understanding of fundamental principles, as well as provide strategies for using applied forces to mature engineered tissue.
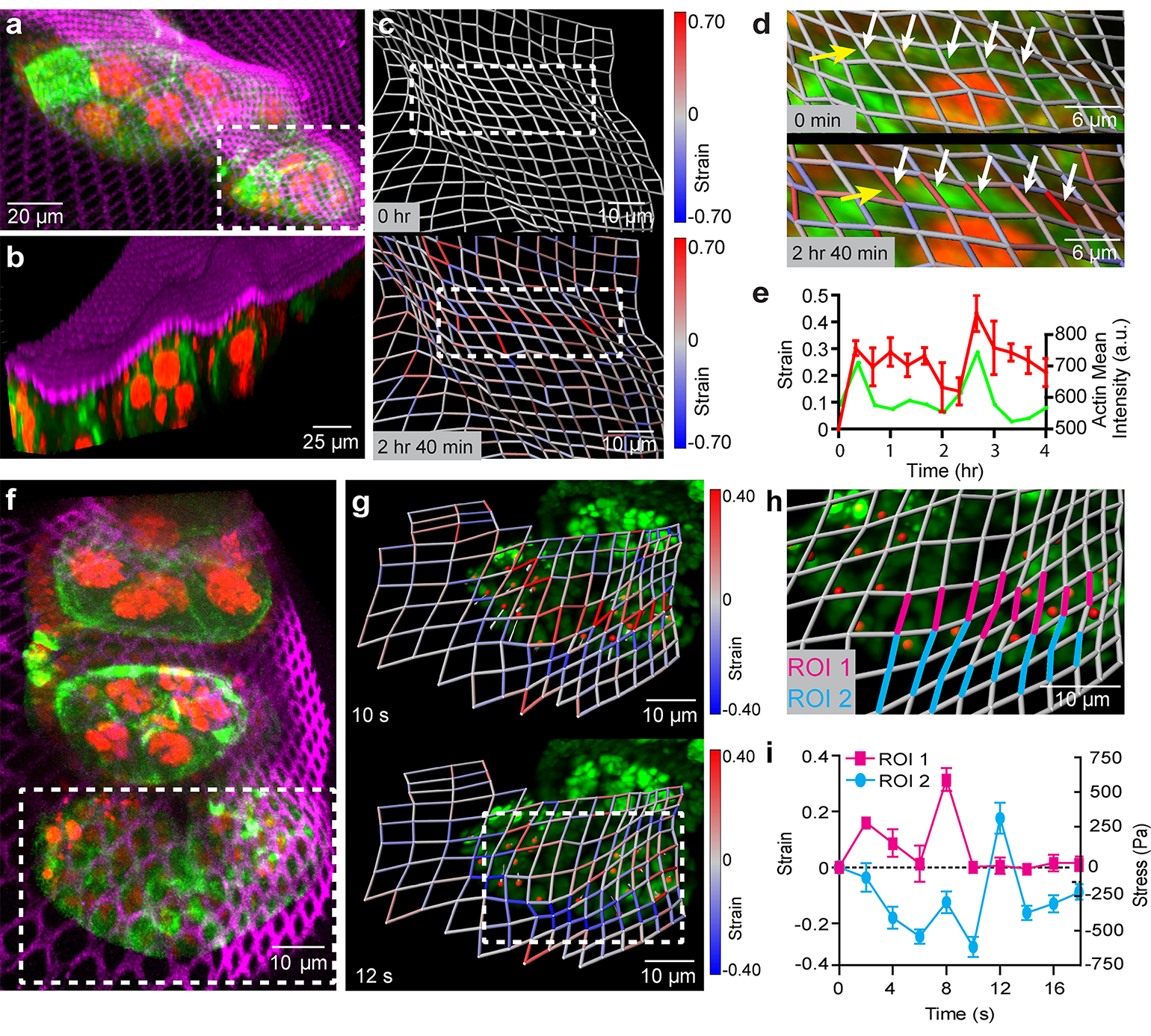
Source: Carnegie Mellon University
The NMBS utilized for 3D strain mapping of drosophila ovariole tissue.
“With the ability to culture and differentiate large quantities of functional cells from induced pluripotent stem cells, a new era of 3D tissue engineering has evolved to reveal the potential of regenerative medicine,” said lead author Shiwarski. “However, researchers are now finding out that, in addition to growth factor driven differentiation processes, the inclusion of mechanical forces is essential to stimulate proper cellular organization and tissue function.”
In their recent publication, Feinberg’s team worked to develop their novel nanomechanical biosensor (NMBS) as a means for measuring and mapping the effect of mechanobiologica l forces on cell movement, differentiation, and tissue growth. The NMBS is a fluorescently labeled mesh made from extracellular matrix protein with individual lattice sections measuring from 2-100 micrometers.
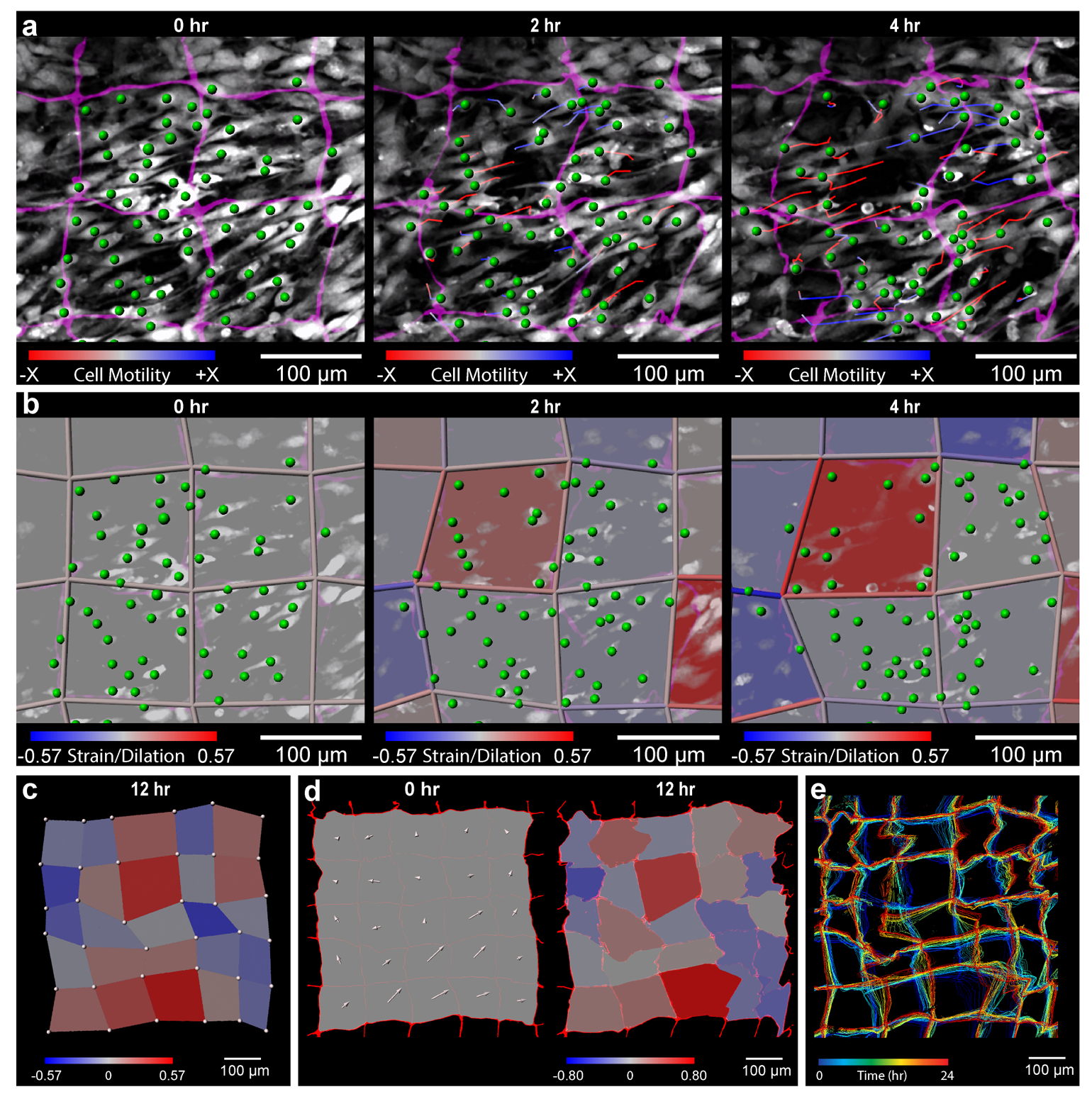
Source: Carnegie Mellon University
The NMBS utilized for 3D strain mapping of drosophila ovariole tissue.
By observing the NMBS lattice in 3D over time, the team was able to track microscopic movement between cells and their environment. Using a series of custom image analysis and segmentation techniques they were then able to convert the cell-induced deformations in the NMBS into a map depicting the strain, directionality, and dynamics of the mechanobiological forces at work. Their method was verified through computational simulation, mechanical testing of materials with known properties, and application of the NMBS onto the surface of cells and developing tissue.
Understanding the deformation that mechanobiological forces impart on developing tissue is crucial to recognizing how a collection of cells come together to form an organ. The cells and extracellular matrix are subjected to forces throughout their growth, shaping how they form and assemble into a cohesive system. If researchers can map the mechanobiological forces at play during development than they can start creating ways to prepare and “train” cells to replicate the natural growth process. As we continue to gain a better understanding of mechanobiological forces and their effect, the NMBS could also potentially prove useful for future research in medicine and cell biology.
We are really excited that the NMBS technology will provide new insights into the role mechanical forces play in a wide range of biological phenomena.
Adam Feinberg, Professor, Biomedical Engineering and Materials Science and Engineering
“We are really excited that the NMBS technology will provide new insights into the role mechanical forces play in a wide range of biological phenomena, from diseases such as hypertension to the formation of new tissues and organs during development. Ultimately, we hope that this knowledge will help lead to new and improved therapies,” said Feinberg.
Much like 3D printing techniques also being developed in Feinberg’s lab, the NMBS is a new tool developed to overcome one of the major hurtles standing between modern healthcare and a near endless supply of lab grown replacement organs. Though we may still be decades from realizing that goal, important tools such as this continue to advance tissue engineering for regenerative medicine and change the way we understand the process of organ development.
