Synthetic muscle gets its punch from design method
Carnegie Mellon researchers design building blocks for synthetic muscle using computational method.
Each time you flex your bicep, millions of molecular motors work together in a complex process inside your muscle. These motors—called myosin—are chemically-powered proteins. Combinations of them perform different muscular functions like maintaining a heartbeat or lifting weights.
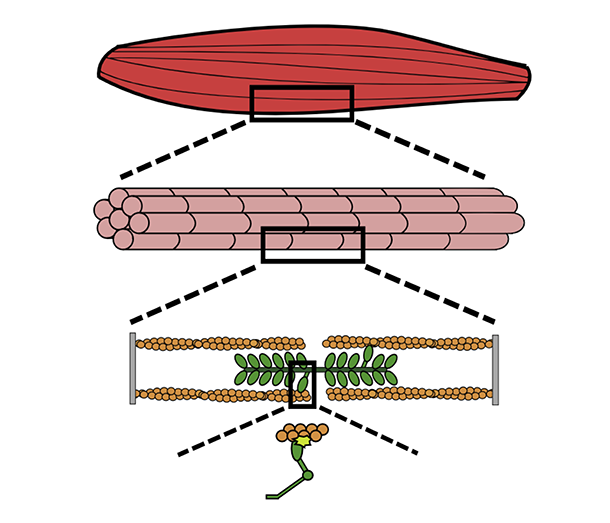
Source: Paul Egan, Carnegie Mellon University
Muscle is made up of molecular motors called myosin that form complex combinations to perform specific functions, such as powering muscle contraction.
In order to develop synthetic muscles for applications in regenerative medicine or robotics, scientists must understand which combination of myosin produces each desired action. This would require a labor-intensive process of nanoscale trial and error that could take years in the laboratory.
Researchers at Carnegie Mellon University’s College of Engineering have taken a multidisciplinary approach to solving this problem. By coupling computational design search methods with biomechanical fundamentals, they created a formal approach for designing myosin systems with specific properties. The findings were published in Proceedings of the National Academy of Sciences this week.
It is similar to using an erector set with nanometer sized proteins to build a moving system.
Philip LeDuc, Professor, Mechanical Engineering, Carnegie Mellon University
The team developed a new computational model that designs systems where multiple myosin types operate together and demonstrates the benefits over different single types of myosin. Laboratory experiments then confirmed the computational predictions.
“This computational method will help us to understand muscle better through one of its building blocks, myosin, and help us toward building synthetic muscle in the future. It is similar to using an erector set with nanometer sized proteins to build a moving system,” said Philip LeDuc, a professor of mechanical engineering.
These findings, which represent a unique collaboration between engineering disciplines, could further impact future applications for understanding and treating myosin-related diseases and developing new approaches for motor molecule-based technologies.
“This work demonstrates that the interface between fields can yield novel approaches to research and new findings that would be difficult to achieve with any one perspective,” said Jonathan Cagan, professor of mechanical engineering.
“We are merging computational mechanical design—which has been used to design a variety of more traditional systems like automobiles and architecture—with biology,” said LeDuc.
More information: “Robust mechanobiological behavior emerges in heterogeneous myosin systems,” Proceedings of the National Academy of Sciences, DOI: 10.1073/pnas.1713219114
